There are many types of intermolecular forces (IMFs).
London Dispersion Forces ✨ ✨ ✨
- These forces do not involve polar molecules.
- AKA Induced dipole - induced dipole forces
- All molecules experience LDFs (since all have an induced dipole)
- Examples: Br₂, I₂
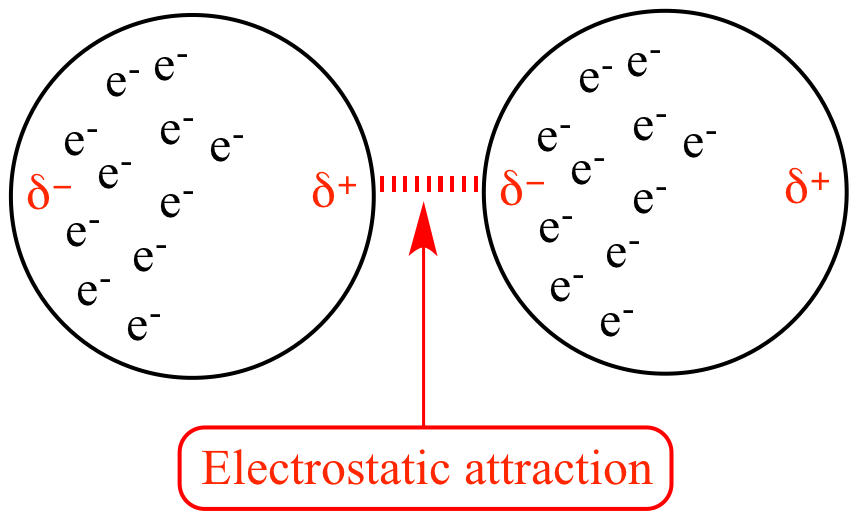
Note that in the image above, these are induced dipoles - basically electrons being in random places creates incredibly weak dipoles (This is why LDFs are so weak!)
Dipole-Dipole Forces ✨ ✨ ✨
- These forces do involve polar molecules.
- Examples: CH₃Cl, PCl₃
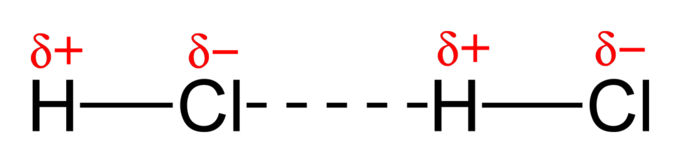
Hydrogen Bonding ✨ ✨ ✨
- These forces involve hydrogen and nitrogen, oxygen, or fluorine. You can remember this through the mnemonic device NOF.
- Examples: H₂0, NH₃, HF
- Fun fact: Hydrogen bonding is why water has so many unique properties!
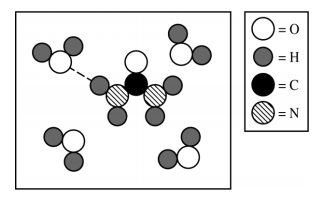
Source: 2019 AP Exam Scoring Guides
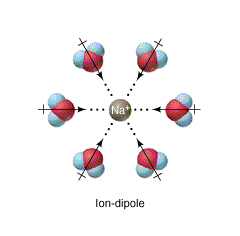
Ion-Dipole Forces ✨ ✨ ✨
- These forces involve both polar molecules and ions, and are typically salts in water.
- Examples: NaCl in H₂0, NBr in H₂0
It is important to note that ionic bonding is absent from this list of IMFs. Technically speaking, ionic bonds are not intermolecular forces due to the lack of covalent bonds.
- As strength of the bond/force increases, so do melting and boiling point.
- In order from weakest to strongest forces:
London dispersion forces, Dipole-dipole forces, Hydrogen bonding, Ion-dipole forces, Ionic bonding.